We reviewed the class before for the test.
Thursday, May 17
Test Day
We are currently writing our unit test on Organic Chemistry. Please do not disturb
This compound contains a carboxylic acid, alcohol, benzene, and amine group. It's aromatic.
We reviewed the class before for the test.
We reviewed the class before for the test.
Monday, May 14
May 14th - An Esterfic Lab
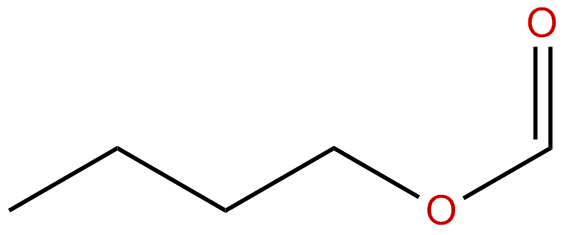
Today, we did an esterfication lab.
There's not much to talk about. Remember that blog on esterfication? It's pretty much the same thing. Except, instead of writing out esterfication equations, WE ACTUALLY DID THEM!
Our first step was to take the carboxylic acid and the alcohol and mix 'em. After, we added some sulfuric acid (the special ingredient) and heated the solution close to a boil. Our esterfication reactions produced an ester and water. The esters had special properties; they smelt like everyday objects! Esters are responsible for the smells of banana, orange, rum, perfume, and more! Here's one that we made:
There's not much to talk about. Remember that blog on esterfication? It's pretty much the same thing. Except, instead of writing out esterfication equations, WE ACTUALLY DID THEM!
Look how much fun esterfication can be?
Our first step was to take the carboxylic acid and the alcohol and mix 'em. After, we added some sulfuric acid (the special ingredient) and heated the solution close to a boil. Our esterfication reactions produced an ester and water. The esters had special properties; they smelt like everyday objects! Esters are responsible for the smells of banana, orange, rum, perfume, and more! Here's one that we made:
There's not much else to say. If you'd like to learn more about esters, view our previous post.
See you on t-day!
Posted by Michael.
Thursday, May 10
Yummy Esters
An ester
is an organic compound made by replacing the hydrogen of an acid by an alkyl or
other organic group. For our studies, it is formed through the combination of a
carboxylic acid and an alcohol. The hydroxyl groups combine to form water and
our ester. The ester has a double bonded oxygen as well as a single bonded one
to the same carbon. It has the ending
–anoate.
The ‘parent’ chain is where the oxygen atoms want to give a hug.
To name an
ester:
The
hydrocarbon chain directly attached to the carbon side of the COO group has the
ending –anoate. Then normally name the 'side' chain.
Esterification
is the reaction of a carboxylic acid and an alcohol to form water and an ester.
In order for them to be formed, we need an inorganic acid. After some magical
chemical reactions, we are left with the pleasant smell of the ester. A few
smells are:
Ethyl methanoate:
rum *yum*
Methyl
butanoate: pineapples
Pentyl
ethanoate: banana
Now it’s
time to draw. Draw:
Butyl
methanoate
Esterification: Label the diagrams and determine a
relationship between the reactants and products.
The reactants are methanol and butanoic acid.
The products are water and methyl butanoate.
The relationship: butanoate = butanoic acid / methanol =
methyl.
In this way, the alcohol forms the ‘side chain’ and the
carboxylic acid forms the parents.
Friday, May 4
Amines, Amides, Nitro, and Esters
Today we
learned four more functional groups. Here is a quick overview of them:
Amines:
contain nitrogen. Primary, secondary, or tertiary amines (one/two/three carbon
chains). Alphabetical ordering.
Amides: CONH2
is the amide group. Ending is –(an)amide. Ie. Benzamide.
Nitro:
contains NO2 which has resonance. It is not a parent chain.
Esters:
contain a =O and a –O on the same carbon.
And there
you have it! Let’s try identifying some now.
Amines:
Name:
This
compound is phenyl amide. Another common name is aminobenzene.
Amines can
be named in many ways. You can have methylamine, methanamide, or aminomethane
and they are all the same. You can sometimes have the amine be a parent chain.
Amides:
Amides contain a double bonded oxygen and nitrogen both attached to a carbon.
Notice in the diagram there are NH2 molecules. Other than hydrogens
in those spots, we can have more carbon chains. This functional group contains
CONH2; it’s the amide group.
Inspect:
Molecules
containing amides end in –amide. The prefix is simply the number of carbons
(including the one in the amide group).
Nitro:
functional group if NO2. It has alternating single and double bonds.
It is not used as a parent chain. It has a simple side chain called ‘nitro’ and
is preceded in a molecule with a locant.
Name:
It is: 2-methyl,
1,3,5 trinitrobenzene. It is also called trinitro tuolene, or in other words,
TNT.
Monday, April 30
April 30th - Naming Groups... Continued
Today, we continued our work with naming groups. Last class,
we went over the rules for naming. This class we mostly did examples.
One important addition: carbon chains without functional
groups are often abbreviated as R. For example, R-OH would be alcohol.
Here are the examples we went through:
Ex.) Draw: 2, 3 diphenyl 3 ethyl 1, 5 pentadiol
To draw this compound, first draw the parent chain. Afterwards, attach two OH groups to carbons 1 and 5 on the parent chain. Finally, add two benzene side chains and an ethyl at the appropriate places.
Ex.) Draw: Phenol
This is a special compound. Phenol consists of a benzene attached to a single OH group.
We also learned about aldehydes. Like ketones, aldehydes
have an oxygen double bond. However, in this case, the oxygen is bonded on the
end. The suffix for the compound is ‘-al’.
The simplest form is methanal, which is better known as
formaldehyde.
We also started our study of other functional groups.
The first one we learned of is carboxylic acid.
For carboxylic acids, there is a double bonded oxygen (an
aldehyde) and an alcohol functional group on the last carbon. The suffix for this
compound is ‘-ioc acid’.
Ex.) Draw: 3 chloro 2 methyl butanoic acid
That’s it for today’s lesson. Next class, we can expect to continue learning about functional groups.
Here's the vid:
Posted by Michael.
Subscribe to:
Posts (Atom)