An ester
is an organic compound made by replacing the hydrogen of an acid by an alkyl or
other organic group. For our studies, it is formed through the combination of a
carboxylic acid and an alcohol. The hydroxyl groups combine to form water and
our ester. The ester has a double bonded oxygen as well as a single bonded one
to the same carbon. It has the ending
–anoate.
The ‘parent’ chain is where the oxygen atoms want to give a hug.
To name an
ester:
The
hydrocarbon chain directly attached to the carbon side of the COO group has the
ending –anoate. Then normally name the 'side' chain.
Esterification
is the reaction of a carboxylic acid and an alcohol to form water and an ester.
In order for them to be formed, we need an inorganic acid. After some magical
chemical reactions, we are left with the pleasant smell of the ester. A few
smells are:
Ethyl methanoate:
rum *yum*
Methyl
butanoate: pineapples
Pentyl
ethanoate: banana
Now it’s
time to draw. Draw:
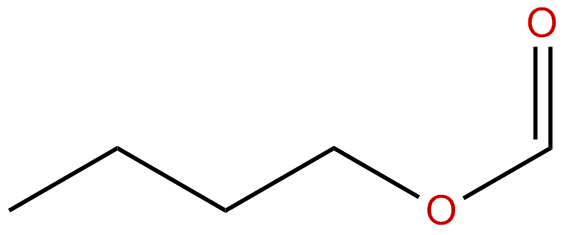
Butyl
methanoate
Esterification: Label the diagrams and determine a
relationship between the reactants and products.
The reactants are methanol and butanoic acid.
The products are water and methyl butanoate.
The relationship: butanoate = butanoic acid / methanol =
methyl.
In this way, the alcohol forms the ‘side chain’ and the
carboxylic acid forms the parents.



Best ias coaching in bangalore
ReplyDelete.www.globalias.in