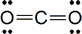Today’s lesson was about the naming of compounds. The IUPAC (International Union of Pure and Applied Chemistry) provides guidelines on how to name compounds. The ones we looked at today included:
- Ionic
- Binary Ionic
- Polyatomic Ions
- Hydrates
- Molecular Compounds
- Acids/Base
Zn2+: This contains an ion charge, and is called “zinc ion”.
MgCl2: The subscript refers to the number of ions.
Hydrogen can be both a hydride and hydrogen ion.
So let’s begin. First of all, we should determine the type of bond. Let’s start off with ionic and covalent bonds.
We can determine the type of bond between ionic and covalent using electronegativity difference. To do so, we have to follow these three steps:
- Look up the electronegativity of both atoms involved in the bond.
- Subtract the smaller electronegativity from the larger.
- Use the electronegativity difference to determine the type of bond.
- 1.7 – 3.3 Ionic Bond
- 0.3 – 1.7 Polar Covalent Bond
- 0.0 – 0.3 Non-polar Covalent Bond
Now that we veered off topic, let’s talk about ionic bonds. Ionic bonds occur between a metal and a non-metal. The name of the metal comes first, followed by the non-metal, which changes its suffix to “-ide”. The anion (negative charge) does not change its name when referring to complex ions. We can cross the ion charges for the proper composition of the ions to have a net charge of zero. Remember, in ionic bonding, electrons are shared. One example is sodium and chlorine. We get NaCl.
Some elements can form more than one ion. This makes them multivalent. When writing the written version of the compound, we have to state which charge is used using roman numerals. An example is FeO. We have to write: iron (II) oxide.
Previously, before the IUPAC existed, there was the classical Latin naming system. There was the suffix –ic which meant large charge, and –ous which meant smaller charge. This means that this method works only with ions that only have two possibilities. Classical names include:
- Ferr – Iron
- Cupp – copper
- Mercur – Mercury
- Stann – Tin
- Aunn – Gold
- Plumb – Lead
- Wolf – Tungsten
- Argent – Silver
An example is plumbous sulphide. The formula would be PbS.
Next we have complex ions, which are held together by covalent bonds and participate in ionic bonds. They follow the same rules as ionic bonds. Be careful though, if a charge applies to them, brackets are needed.
Example: ammonium oxide. The answer is (NH4)2O.
Before we move on, we need to cover covalent bonds. They are simple, and use the prefixes mono, di, tri, tetra, etc. to tell us of the exact proportions, unlike ionic bonds, which give us a ratio. The prefix mono is used only on the second element.
Example: S2O4 The answer is disulphur tetraoxide.
Some compounds can form lattices that bond to water molecules. These are called hydrates. These crystals contain water inside them which can be released by careful heating. To name hydrates:
- Write the names of the chemical formula.
- Add a prefix indicating the number of water molecules.
- Add hydrate after the prefix.
That`s about it! Here's a video for additional review.
Posted by Andrew.