As you can see, the Lewis diagrams hold the same number of electrons as the last shell of the Bohr diagram.
When an electron exists by itself in one of the four orbitals, it is called a bonding electron. When two electrons are in the same orbital, they form what is called a lone pair.
We can also draw Lewis diagrams for compounds and ions. In covalent bonds (bonds formed by sharing electrons), we can create a diagram by:
- Determining the number of valence electrons for each atom in the molecule
- Configure the atoms so that the valence electrons are shared to fill each orbital and form a stable octet (full shell of 8 electrons)
CO2 is made up of:

To pair up the electrons, we start with carbon. The atom that is the least stable in the compound is the central atom. We also must include two oxygen atoms into the equation. We bond the electrons, like so:
The compound takes this form, which is our final Lewis structure:
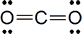
In the above diagram, the dashes represent pairs of electrons. There are two lines between the oxygen particles and the carbon particle, which represent a double bond. These lines apply to both particles; both oxygen particles and the carbon particle have eight electrons. The molecule is stable and there are no leftover valence electrons. Here's a video that shows the process in detail:
We can also draw Lewis diagrams for ionic compounds (bonds formed by transferring electrons). We can create a diagram by:
- determining the number of valence electrons in the cation (positive ion)
- moving these electrons to the anion (negative ion)
- drawing square brackets around the metal and non-metal
- writing the charges outside the brackets (because each became an ion by losing/gaining electrons)
NaCl is made up of:
To pair up the electrons, we take the extra electron from sodium and transfer it to the empty space in chlorine's valence shell. We draw square brackets around each, and write the charges outside the brackets. This is the final product:
It becomes stable; the valence shells are full! We have just successfully drawn a Lewis diagram for sodium chloride!
Posted by Michael.







No comments:
Post a Comment