After our two week break, we're going to dive right into our next topic: empirical formulas. Empirical formulas are the simplest formulas of a compound. They exist in contrast to molecular formulas. Here are a few examples:
Empirical Molecular
C4H9 C8H18
NO2 N2O4
P2O5 P4O10
Another way of saying it is that the empirical formulas are simply the molecular formulas in lowest terms. Empirical formulas only show a ratio of the respective elements in a compound; molecular formulas show the actual number of atoms that bond to form a compound.
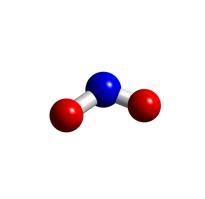
Note: distinguishing between empirical formulas and molecular formulas can be tricky. For example, take the molecular formula N2O4. It looks like this:
The name of this compound is dinitrogen tetraoxide. The empirical formula for the same compound is NO2, which is nitrogen dioxide. It looks like this:
In this case, you happen to be dealing with completely different compounds.
To determine an empirical formula, we need to know the ratios of each element in a compound. To determine the ratios, we can construct a chart, like the one in the following example:
Ex.) Determine the empirical formula of a compound containing 0.928g of gallium and 0.412g of phosphorus.
As you can see, in the first column is the name of the element/atom. In the second, we have the mass, which is usually given in the question. If not, you can figure it out through simple subtraction. In the third, we have the molar mass. This can be found in the periodic table for each element. Next, we have the moles. Using the conversions we learned earlier in this unit, we can figure 'em out:
0.928g x mol/69.7g = 0.0133 mol of gallium
0.412g x mol/31.0g = 0.0133 mol of phosphorus
Oddly, in this example, we end up with the same number of moles for both compounds.
The fifth column is where we find out number of gallium atoms and phosphorus atoms in the compound. To find this out, we divide the mole for gallium by the smallest mole out of all the calculations we made in column four. We would then do the same for phosphorus. Strangely, in this compound, the ratio would be 1:1 because the moles are equal. 0.0133/0.0133 = 1. In this column, we put 1:1.
The sixth column would contain the lowest term ratio of atoms in the compound (remember: it is the empirical formula!), but in this case we have already obtained the lowest term ratio. We're done! The compound is GaP, or Gallium phosphide.
Note: in some cases, you may get a ratio that ends up being 1.33 or 1.66. In this case, you would need to multiply it so it becomes a whole number. You would multiply both of those by 3, so your new ratio would be 4 or 5. It's simple!
I hope you enjoyed the lesson. If you want more examples, here are a few great videos that include all sorts of variations of this process:
Posted by Michael.



No comments:
Post a Comment