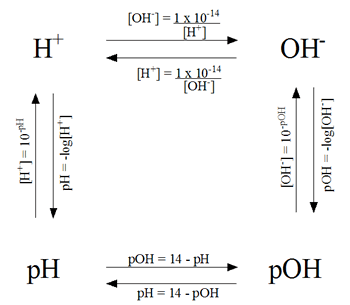
Actually, today’s lesson was very interesting! We were able to determine the pH of the a lake before and after acid rain fell on it. To do this, we needed the following equation:
pH = - log [H+]
pOH = - log [OH-]
Other equations include:
pH + pOH = 14
[H+][OH-] = 10-14
Ex. Simple. HCl à H+ + Cl-. Therefore, 0.150L (0.30mol/1L)(1/1)(1/0.150L) = 0.30 M H+
pH = - log [0.30M] = 0.52!
Ex. After this amount falls on a lake of 2000mL of 0.02M NaOH, what is the pH of the lake?
Since we have two reactants, we first need to find the limiting reactant.
There are 0.045 mol HCl present. There are 0.040 mol NaOH present.
HCl + NaOH à NaCl + H2O
There are 0.045 mol NaOH needed. Therefore, NaOH is the limiting reactant.
There are 0.040 mol HCl needed. After the reaction, we are left with 0.005 mol HCl.
Dissociate it! HCl à H+ + Cl-
There will be 0.005 mol H+, and since our solution has 2.150L, [H+] is 0.0023255814.
pH = 2.6! That’s pretty acidic.
And that’s it. So remember, when performing acid-base reaction with two reactants, ‘follow the following’ steps.
Find the LR
Determine how many moles of excess reactants are left
Finding out number of moles of H+ or OH-.
Finding the concentration by dividing by volume of [H+ or OH-].
Using -log.
Fun fact: Ka is a ratio of dissociated to un-dissociated acid. Ie. [H+][F-] / [HF]
Posted by Andrew.

No comments:
Post a Comment