Today’s class was all about the Bohr Model of the Atom. We learned that Niels Bohr was a Danish physicist who made fundamental contributions to our understanding of matter through his explanation of the atomic structure and his research on quantum mechanics. We study his atomic theory in class. His model works beautifully, but with only one element. That element is hydrogen. Other than that, his theory isn't perfectly. Nevertheless, it is simple enough that we can learn it.
Niels Bohr expanded on Ernest Rutherford’s theory. Recalling from the last lesson, Rutherford stated that the atom consisted of a positively charged nucleus with negatively charged electrons orbiting around it. There was a phenomenon Rutherford could not explain. If the nucleus is positive and the electron is negative, why won’t the electron spiral into the nucleus and create a neutron?
We all know that we can observe the visible spectrum of light when it passes through a prism. Niels Bohr also observed this. He, however, recorded the distinct lines of colour when a specific element was passed through a prism. For example, the image below illustrates the line spectrum produced with the element hydrogen:
When matter is heated, it emits light. Using this knowledge, Bohr based his model on the energy emitted by different atoms. In class, we also touched on black body radiation. This is radiation that a body emits when heated.
Each element has a unique line spectrum that differs from the spectrum of other elements. This property makes it possible for physicists and astronomers to determine what element is present in a celestial body or in space.
When matter is heated, it emits light. Using this knowledge, Bohr based his model on the energy emitted by different atoms. In class, we also touched on black body radiation. This is radiation that a body emits when heated.
Next, explored PHET simulations. In one simulation, we noticed a strange particle flying off when an electron moved from high energy level to a low one. We learned that when an electron changes energy levels, the result is visible light (in the form of photons or waves). We also learned that the colour emitted when moving from one shell to another was always consistent.
For example, let’s say we have an atom. We add enough energy to make the electron move from the first shell to the fourth shell. When the electron moves from the fourth shell back to the first, it will always release 'blue'. If the electron moved from the second shell to the first, it would release 'red'. It is important to note the colour emitted. According to the electromagnetic spectrum, blue has more energy than red. This means that blue has a higher frequency than red (frequency can be described as the number of waves that pass a fixed place in a given amount of time). In addition, if an electron moves up 10 units, it will fall back ten. If it initially moved down 6, it will go back the remaining 4.
For example, let’s say we have an atom. We add enough energy to make the electron move from the first shell to the fourth shell. When the electron moves from the fourth shell back to the first, it will always release 'blue'. If the electron moved from the second shell to the first, it would release 'red'. It is important to note the colour emitted. According to the electromagnetic spectrum, blue has more energy than red. This means that blue has a higher frequency than red (frequency can be described as the number of waves that pass a fixed place in a given amount of time). In addition, if an electron moves up 10 units, it will fall back ten. If it initially moved down 6, it will go back the remaining 4.
The emitted light can exist as a photon. Like an electron, it has both particle and wave properties. A photon has no mass or electric charge. It does, however, have energy, which depends on its wavelength. Blue would have more energy than red.
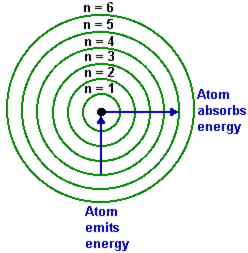
Niels Bohr proposed that electrons occupy certain shell in an atom. Rutherford was unable to describe the location of the electrons. Niels Bohr said that electrons move in energy levels around the nucleus. Each orbit represents the amount of energy the electrons in them contain. When energy is absorbed, they move to higher energy levels. When the electrons move back from this excited state, they release energy. An interesting point to note is that only photons of the same colour will bump an electron up to higher levels. Photons of other frequencies will pass right through the atom.
Some important points about the Bohr Model of the Atom include:
1. The circumference of the orbit must equal wavelength, twice the wavelength, three times, and so on
2. Other orbits produce destructible interference of the waves
3. Electrons don’t travel around the nucleus in a circle. They take form in a standing wave that surrounds the nucleus entirely
Today we started exploring the quantum world. Though it seems unbelievably random and we seem to barely make sense of it, we are eager to learn more.
Posted by Andrew.


No comments:
Post a Comment