This class, we continued our study of the Bohr model. We began the class by getting our test results back and receiving our blog marks (for the first unit). Afterwards, we jumped right into to main topic of the day.
To summarize the last lesson:
- Electrons exist in orbitals
- When they absorb energy, they move to a higher orbital
- As they fall from a higher orbital to a lower one, they release energy as a photon (light)
In class, we also looked at a PhET simulation, which can be found here. The simulation looks at the structure of an atom. You can add electrons, neutrons, and protons to manipulate atoms. You can even see how adding different particles changes the characteristics of the atom! Neat!
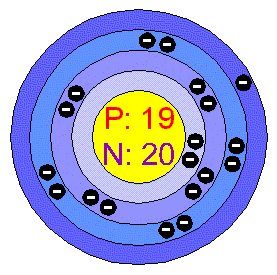
As I mentioned earlier, electrons occupy shells which are divided into separate orbitals. Each orbitals can only contain a specific number of electrons. Starting from the nucleus and going outward, the pattern is:
- The first orbital can hold two electrons
- The second orbital can hold eight electrons (called an octet)
- The third orbital can hold eight electrons (called an octet)
- The fourth orbital can hold eighteen electrons
To draw a Bohr model diagram, start with the nucleus. Include the number of protons (atomic number) and the number of neutrons (atomic number subtracted from atomic mass). Then, following the pattern, write the number of electrons that occupy each shell. In the first shell of a fluorine atom, there will be two, since two is the maximum. In the second, there will be seven, because the number of total electrons must be equal to the number of protons in an atom.

Posted by Michael.




No comments:
Post a Comment